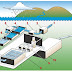
WATER TREATMENT
Most municipalities must use a source of water in which the probability of pollution is rather high. Certainly, all our natural rivers and lakes and even the water stored in most reservoirs may be subjected to pollution, and generally cannot be considered safe for drinking purposes without some forms of treatment. The type and extent of treatment will vary from city to city, depending upon the conditions of
the raw water. Treatment may comprise various processes used separately or in combinations, such as storage, aeration, sedimentation, coagulation, rapid or slow sand filtration, and chlorination, or other accepted forms of disinfection.
When surface waters serve as a municipal water supply, it is generally necessary to remove suspended solid, which can be accomplished either by plain sedimentation or sedimentation following the addition of coagulating chemicals. In the water from most streams that are suitable as a source of supply,
the sediment is principally inorganic, consisting of particles of sand and clay and small amount of organic matter. In this water there will also be varying numbers of bacteria, depending upon the amount of
bacteria nutrients, coming from sewage or other sources of organic matter, and upon the prevailing
temperature. Many of the bacteria may have come from the soil and, as a result, during a season of high turbidity when there is a large amount of eroded soil in the water, the bacterial count from this source
may be relatively high. If the organisms are derived from sewage pollution, the number will be highest during periods of low flow when there is less dilution, and at this time the turbidity will, in general, be low. The amount of sediment may vary a great deal from one river to another, depending upon the
geological character of the various parts of the drainage system. The size of the suspended particles can also vary greatly. In some waters the clay particles may be extremely fine, in fact, they may be smaller
than bacteria. The time required for satisfactory sedimentation differs for different waters, and generally must be established by actual experiments. Some waters can be clarified satisfactorily in a few days,
while others may require weeks or months. As far as total weight of sediment is concerned, the bulk of it
is probably removed in a few days, but this may not bring about a corresponding change in the appearance of the water, since the smaller particles may have greater influence than the large ones upon
the apparent color and turbidity. When plain sedimentation is used primarily as a preliminary treatment, a high degree of clarification is not needed and, as a result, shorter periods of settling are adequate.
After flocculation treatment, water is passed through beds of sand with diatomaceous earth to accomplish sand filtration. As we mentioned previously, some protozoan cysts, such as those of G.lamblia, appear to be removed from water only by such filtration treatment. The microorganisms are trapped mostly by surface adsorption in the sand beds. They do not penetrate the tortuous routing of the sand beds, even through the openings might be larger than the organisms that are filtered out. These sand filters are periodically backflushed to clear them of accumulations. Water systems of cities that have an exceptional concern for toxic chemicals supplement sand filtration with filters of activated charcoal (carbon). Charcoal has the advantage of removing not only particulate matter but also some dissolved organic chemical pollutants.
Before entering the municipal distribution system, the filtered water is chlorinated. Because organic matter neutralized chlorine, the plant operators must pay constant attention to maintaining effective levels
of chlorine. There has been some concern that chlorine itself might be a health hazard, that it might react
with organic contaminants of the water to form carcinogenic compounds. At present, this possibility is considered minor when compared with the proven usefulness of chlorination of water.
One substitute for chlorination is ozone treatment. Ozone (O3) is a highly reactive form of oxygen that is formed by electrical spark discharges and ultraviolet light. (The fresh odor of air following an electrical storm or around an ultraviolet light bulb is from ozone). Ozone for water treatment is generated electrically at the site of treatment. Use of ultraviolet light is also a possible alternative to chemical disinfection. Arrays of ultraviolet tube lamps are arranged in quartz tubes so that water flows close to the lamps. This is necessary because of the low penetrating power of ultraviolet radiation.



.jpg)






0 comments:
Post a Comment