ISOLATION AND PURIFICATION OF SUBSTANCE
 Practical chemistry includes many special techniques for the isolation and purification of substances. Some substances occur very nearly pure in nature, but most materials are mixtures, which must be separated or purified if pure substances are desired, and most manufactured materials also require purification.
Practical chemistry includes many special techniques for the isolation and purification of substances. Some substances occur very nearly pure in nature, but most materials are mixtures, which must be separated or purified if pure substances are desired, and most manufactured materials also require purification.The separation of two different phases is often rather easy. Particles of a solid phase mixed with a liquid phase may be separated from the liquid by filtration. Often the solid is present because it has been produced from solution in the liquid by a chemical reaction or by change in conditions/such as by cooling/ the solid is then called the precipitate. The precipitate is removed by pouring the mixture on a folded filter paper in a funnel. The liquid/ called the filtrate/ runs through, and the grains of precipitate/
the residue/ are retained, unless they are too small. Ordinary filter paper contains pores about 0.001cm in diameter, and smaller particles pass through.
A precipitate may also be removed by letting the suspension stand quietly until the precipitate has settled to the bottom of the container under the influence of gravity. The supernatant liquid can then be poured off. This process of pouring off is called decantation.
The process of settling can be accelerated by the use of centrifugal force, in a centrifuge. Ordinary centrifuges produce forces of the order of 100 or 1,000 times that of gravity. Supercentrifuges have been built which give forces over 100,000 times as great as that of gravity.
Two liquid phases may be conveniently separated by use of a special device, the separatory funnel.
A dropper may also be used for this purpose.
An impure substance may often be purified by fractional freezing. The impure liquid substance is cooled until part of it has crystallized, and the remaining liquid, which usually contains most of the impurities, is then poured off, leaving the purified crystals.
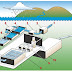
A liquid can be purified by distillation in a still. The liquid is boiled in a flask or some other container, and the vapor is condenser, forming a liquid distillate, which is collected in a receiver. The first portions/fractions/ of the distillate tend to contain the more volatile impurities, and the residue in the flask tends to retain the less volatile ones. Stills so special design have been invented, which are very effective
in separating liquid mixtures into their components.


.jpg)






This comment has been removed by the author.
ReplyDelete